

Protection contre les méduses : Medusyl
TEST SUR L'EFFICACITE D'UN INHIBITEUR DE PIQÛRES DE MEDUSES SUR DES SUJETS VOLONTAIRES EN BONNE SANTE
(Commercialisé sous le nom de MEDUSYL)
Diaporama MEDUSYL
Film MEDUSYL
Tests sur MEDUSYL en mer (version anglaise)
Alexa Boer Kimball, MD, MPH ; Karina Zuelma Arambula, BA ; Arlen Ray Stauffer, MD ; Valeh Levy, MD ; Valerie Weaver Davis, MD ; Michael Liu, MD ; Wingfield Ellis Rehmus, MD, MPH ; Amit Lotan, PhD ; Paul S. Auerbach, MD, MS From the Department of Dermatology (Drs Kimball, Arambula, and Rehmus) and Division of Emergency Medicine (Drs Liu and Auerbach), Stanford University School of Medicine, Stanford, CA ; the Bert Fish Medical Center, New Smyrna Beach, FL (Drs Stauffer, Levy, and Davis) ; andNidaria Technology, Kinarot, Zemach, Jordan Valley, Israel (Dr Lotan).
Objectif : Evaluer les effets protecteurs d'un inhibiteur de piqûres de méduses formulé dans une lotion solaire VS une lotion solaire conventionnelle contre les méduses Chrysaora Fuscescens et Chiropsalmus Quadrumanus
Méthodes : 24 sujets en bonne santé sur deux sites de recherche distincts furent désignés pour recevoir l'inhibiteur de piqûres sur un avant bras et un écran solaire conventionnel sur l'autre en protocole « aveugle ».
Les sujets furent piqués à l'aide de tentacule de méduses sur chaque bras pendant 60 sec. L'érythème et la douleur sont alors mesurés à 15 min d'intervalle pendant un temps total de deux heures.
Résultats : Dans le groupe Fuscescens, les 12 avants bras traités à la lotion solaire conventionnelle présentent des érythèmes et tous les sujets notent de façon subjectives des inconforts ou douleurs. En comparaison, aucun avant bras traité avec la lotion inhibitrice ne présente objectivement d'érythème. Deux sujets relèvent un petit inconfort.
Dans le groupe Quadrumanus, un inconfort a été relevé chez 3 patient traités avec l'inhibiteur alors que 10 patients sur 12 se plaignent avec le placebo.
L'érythème a été noté chez un sujet traité avec l'inhibiteur contre 9 avant bras traités au placebo.
Conclusions : L'inhibiteur de piqûres de Méduses a prévenu les symptômes du groupe Fuscescens sur 10 sujets sur un total de 12 et diminué la douleur de piqûre chez les deux sujets restant.
L'inhibiteur de piqûres de Méduses a aussi empêché les piqûres les plus sévères du groupe Quadrumanus chez la plupart des sujets.
L'inhibiteur de piqûres de Méduses n'élimine pas les piqûres de Fuscescens ou Quadrumanus mais REDUIT SIGNIFICATIVEMENT la fréquence et la sévérité des piqûres.
INTRODUCTION
Les envenimations de cnidaires, une classe commune de Méduse qui se trouve un peu partout dans le monde, est un problème qui affecte les gens dans leur occupation récréationnelle ou de travail.
Les méduses Chrysaora Fuscescens, aussi connues sous le nom de Sea Nettle sont des Scyphozoans. On les trouve partout, et diverses espèces sont bien connues par les baigneurs et plaisanciers piqués dans la baie de Chesapeake, le long des côte de Floride ou sur la côte ouest des Etats Unis.
Un contact avec les tentacules provoque habituellement une douleur immédiate suivie par un érythème sur la portion de peau piquée.
La piqûre peut être accompagnée d'une sensation de brûlure et parfois de cloques ou de nécroses. Quoique douloureuses, les piqûres ne sont pas mortelles et se résorbent sans traitement particulier.
En contraste, Chiropsalmus Quadrumanus (Méduse boite ou Guêpe de mer) est un Cubozoan et peut causer des réaction plus sévères incluant douleurs intenses, rougeurs marquées, démangeaisons sur la portion concernée. Cette méduse est considérée comme dangereuse pour les humains et peut constituer une menace avérée, voire mortelle sur des petits enfants. Elle est très répandue dans certains océans bordant les Etats Unis, particulièrement dans le golf du Mexique, en Floride et les long des côtes du Texas.
Le processus venimeux des deux espèces Fuscescens et Quadrumanus se déclenche par le contact avec la peau. Ce dernier amène les cellules venimeuses a délivrer de nombreuses toxines. Le mécanisme venimeux de la méduse consiste dans des millions de cellules urticantes (nematocytes) qui réagissent à divers événements mécaniques et chimiques. Chaque cellule venimeuse consiste en une capsule dense, dans laquelle est replié un harpon ou dard. La pression hydrostatique de 150 Atm est atteinte juste avant la piqûre. La capsule est forcée de s'ouvrir sous une telle pression, permettant alors au dard d'être libéré.
Le harpon pénètre dans la peau d'un humain avec une accélération de 40 000 fois la gravité. Les poisons se répandent dans la peau en une fraction de seconde, faisant de la piqûre de méduse un des mécanismes les plus rapides existant dans la nature. Le venin contient de l'histamine, des agents libérateur d'histamine, et de la Sérotonine.
Plus de 150 millions de personnes sont exposées annuellement au risque des méduses, et ces dernières sont la cause de nombreuses piqûres et même décès chaque année.
Alors que les combinaisons sont un excellent moyen d'éviter tout contact avec la méduse et sont recommandés dans les régions à présence de méduses létales, elles sont beaucoup moins utilisées dans les régions habitées par des espèces moins toxiques.
Afin de fournir une protection pratique et peu coûteuse pour les baigneurs et nageurs, une crème repellent a été formulée. Cette crème a été mélangée a un écran solaire contenant de l'OMC et Oxyde de Zinc qui permet en une seule application de se protéger des piqûres et des coups de soleil.
Cette crème est déjà commercialisée dans beaucoup de pays comme le Japon, l'Espagne, l'Italie, les Etats Unis, Israël et se veut« aide à la prévention des piqûres de méduses » et non prévention à 100%.
L'inhibiteur a été formulé pour inactiver les cellules venimeuses de différentes façons.
Premièrement, l'inhibiteur est hydrophobe et cela empêche les tentacules de se « coller » à la peau et donc de piquer. En second lieu, la crème contient des glycoaminoglycans qui imitent ceux présents sur la méduse, ceci conduisant à la tromper eu égard à la reconnaissance de la proie. En troisième lieu, l'inhibiteur contient un antagoniste qui rend les récepteurs non sélectifs devant les amino acides et sécrétions de sucres faite par la proie ou le peau.
Enfin, le calcium et le magnésium réduisent la pression osmotique intra nématocyte nécessaire a l'expulsion du dard.
Table 1. Results fiom C. fuscescens group comparing severity of reaction (including both pain and clinical signs of reaction) and time to reach reaction after contact with tentacles in inhibitor and placebo treated arms
| Inhibitor | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total contact | Max pain | Max | reaction | Max pain | Max | reaction | |||
| Subject N° | Time (s) | Amount of pain | Time (min) | Amount | Time (min) | Amount of pain | Time (min) | Amount | Time (min) |
| 1 | 45 | 0 | 0 | 0 | 0 | 1 | 15 | 2 | 15 |
| 2 | 30 | 0 | 0 | 0 | 0 | 1 | 60 | 1 | 30 |
| 3 | 30 | 0 | 0 | 0 | 0 | 1 | 30 | 2 | 30 |
| 4 | 30 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| 5 | 30 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| 6 | 30 | 0 | 0 | 0 | 0 | 1 | 30 | 2 | 30 |
| 7 | 45 | 0 | 0 | 0 | 0 | 1 | 30 | 2 | 30 |
| 8 | 45 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 120 |
| 9 | 60 | 0 | 0 | 0 | 0 | 1 | 30 | 1 | 30 |
| 10 | 45 | 0.5 | 15 | 0 | 0 | 1 | 0 | 2 | 30 |
| 11 | 45 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| 12 | 30 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| Median | 37.50 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 15.00 | 1.00 | 30.00 |
| Mean | 38.75 | 0.04 | 1.25 | 0.00 | 0.00 | 1.00 | 22.50 | 1.42 | 36.25 |
| P value | <.01 | <.01 | |||||||
Max. maximum ; Min, minimum.
Table 2. Results from C. qiiadvumawus group comparing severity of reaction (including both pain and clinical signs of reaction) and time to reach reaction after contact with tentacles in inhibitor and placebo treated arms.
| Inhibitor | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total contact | Max pain | Max | reaction | Max pain | Max | reaction | |||
| Subject N° | Time(s) | Amount of pain | Time (min) | Amoun | Time (min) | Amount of pain | Time(min) | Amount | Time (min) |
| 1 | 10 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 30 |
| 2 | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 90 |
| 3 | 20 | 0 | 0 | 0 | 0 | 1 | 15 | 2 | 120 |
| 4 | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 120 |
| 5 | 10 | 0 | 0 | 0 | 0 | 1 | 60 | 1 | 60 |
| 6 | 20 | 1 | 15 | 0 | 0 | 1 | 30 | 2 | 30 |
| 7 | 10 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 8 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 20 | 0 | 0 | 0 | 0 | 1 | 30 | 1 | 30 |
| 10 | 20 | 1 | 15 | 1 | 90 | 0 | 0 | 0 | 0 |
| 11 | 30 | 0 | 0 | 0 | 0 | 1 | 60 | 2 | 60 |
| 12 | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| Median | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| Mean | 15 | 0.3 | 2.5 | 0.1 | 7.5 | 0.83 | 20 | 1 | 47.5 |
| P value | <.05 | <.01 | |||||||
Max. maximum ; Min. minimum.
VERSION ANGLAISE
Efficacy of a Jellyfish Sting Inhibitor in Preventing Jellyfish Stings in Normal Volunteers
Alexa Boer Kimball, MD, MPH ; Karina Zuelma Arambula, BA ; Arlen Ray Stauffer, MD ; Valeh Levy, MD ; Valerie Weaver Davis, MD ; Michael Liu, MD ; Wingfield Ellis Rehmus, MD, MPH ; Amit Lotan, PhD ; Paul S. Auerbach, MD, MS From the Department of Dermatology (Drs Kimball, Arambula, and Rehmus) and Division of Emergency Medicine (Drs Liu and Auerbach), Stanford University School of Medicine, Stanford, CA ; the Bert Fish Medical Center, New Smyrna Beach, FL (Drs Stauffer, Levy, and Davis) ; and Nidaria Technology, Kinarot, Zemach, Jordan Valley, Israel (Dr Lotan).
Objective. To evaluate the protective effects of a jellyfish sting inhibitor formulated in sunscreen lotion vs conventional sunscreen against Chrysaora fuscescens and Chiropsalmus quadrumanus jellyfish.
Methods. Twenty-four healthy subjects at 2 research sites were randomly assigned to receive the jellyfish sting inhibitor (Nidaria Technology Ltd, Jordan Valley, Israel) to one forearm and conventional sunscreen to the other arm in a blinded fashion. Subjects were stung with jellyfish tentacles on each forearm for up to 60 seconds. Erythema and pain were assessed at 15-minute intervals over a 2-hour period.
Results. In the C fuscescens group, all 12 arms pretreated with conventional sunscreen demonstrated erythema, and all subjects noted subjective discomfort. In contrast, no arm pretreated with the jellyfish sting inhibitor had objective skin changes (P < .01). Two subjects noted minimal discomfort in the arm treated with the sting inhibitor (P < .01). In the C quadrumanus group, discomfort was reported in 3 of the 12 inhibitor-treated arms compared with 10 of the 12 placebo-treated arms (P < .05). Erythema was noted on 1 arm treated with the inhibitor and 9 arms treated with the placebo (P < .01).
Conclusions. The jellyfish sting inhibitor prevented sting symptoms of C fuscescens jellyfish in 10 of 12 subjects and diminished the pain of the jellyfish sting in the remaining 2 subjects. The jellyfish sting inhibitor also inhibited the more severe sting of the C quadrumanus jellyfish in the majority of subjects. The jellyfish sting inhibitor does not eliminate the sting from C fuscescens or C quadrumanus jellyfish but significantly reduces the frequency and severity of stings.
Key words : jellyfish, inhibitor, Chrysaora, Chiropsalmus, sting, nematocyst
Introduction
Presented in poster form at the American Academy of Dermatology meeting, San Francisco, CA, March 2003.
Supported, in part, by a grant from USSOCOM to Nidaria Technology Ltd.
Corresponding author : Alexa Boer Kimball, MD, MPH, Director of Clinical Trials, Stanford University Department of Dermatology, 900 Blake Wilbur Dr. Stanford, CA 94305 (e-mail : [email protected]).
Envenomation by cnidarians, a common class of jellyfish found worldwide, is a problem that affects people in both recreational and occupational settings.1
Chrysaora fuscescens jellyfish, also known as sea nettles, are scyphozoans. They are found worldwide, and various species are well known for stinging swimmers and boaters in the Chesapeake Bay,2 along the coastline of Florida, and over a broad expanse of waters along the West Coast of the United States3 (Figure 1). Contact with jellyfish tentacles usually causes immediate pain and blanching followed by erythema and induration at the sting site.4 The sting may be accompanied by a burning sensation and occasionally may result in blisters or skin necrosis.5-6 Although painful, the stings are not life-threatening to humans and typically resolve without treatment.7 In contrast, Chiropsalmus quadrumanus (box jellyfish or sea wasp) is cubozoan and causes more severe reactions including severe pain, redness, and swelling at the site of the reaction3 (Figure 2). This jellyfish is considered to be dangerous to humans and may be of special danger, even life-threatening, to small children.7-8 It is prevalent in certain ocean waters of the United States, primarily in the Gulf of Mexico area and along the coastlines of Florida and Texas.
The stinging mechanism of cnidarians such as Chrysaora and Chiropsalmus is triggered when contact with foreign skin induces stinging cells (cnidocytes) to deliver a variety of toxins. The venom delivery mechanism of jellyfish is composed of millions of stinging organelles (nematocysts) that depend on several mechanical and chemical events.9
Each stinging cell consists of a dense capsule, within which is a highly folded harpoon. Hydrostatic (fluid) pressure of 150 atm is developed within the capsule just before the act of stinging10 (Figure 3 ). The capsule is forced open by this pressure, allowing the harpoon to be released. The harpoon penetrates into the skin of a human or other species with acceleration of up to 40 000 times the force of gravity.1113 The poisons are delivered into the skin within a fraction of a second, making a jellyfish sting one of the most rapid mechanical events found in nature.14 The venom contains histamine, histamine-releasing agents, and serotonin, which are injected into the skin upon penetration.15
Over 150 million people are exposed to jellyfish annually, and jellyfish cause many stings and several deaths each year. Whereas rash guards and stinger suits are excellent in preventing contact with jellyfish and are recommended for use in regions where lethal jellyfish are common, they are not widely used in areas inhabited by less toxic jellyfish. To provide practical and inexpensive protection for recreational and professional swimmers, a repellant cream was formulated. This repellant cream was compounded into a waterproof sunscreen containing octyl methoxycinnamate and zinc oxide, which would allow for single-application protection from both sting and sunburn.
This cream is already commercially available in many countries worldwide, including Japan, Spain, Italy, the United States, and Israel, and is promoted as protection against, not complete prevention of, the sting of most jellyfish. However, no data have been published evaluating its efficacy in preventing jellyfish stings.
The inhibitor was formulated to inactivate jellyfish stinging cells in several ways. First, the inhibitor is hydrophobic and thus prevents the tentacles from making sufficient contact with the skin to sting it. Second, the inhibitor contains glycosaminoglycans that mimic the glycosaminoglycans of the jellyfish bell. Because this bell serves as part of the jelly's self-recognition system, the inhibitor can cause the jellyfish to recognize the user as self and prevents sting. Third, the inhibitor contains a competitive antagonist to nonselective receptors on jellyfish that bind to amino acids and sugar secretions from prey. Finally, calcium and magnesium within the inhibitor block transmembrane signaling channels of the jellyfish and work to reduce the osmotic forces required to create the necessary firing force within the capsule for the nematocyst.
Methods
Twenty-four adult subjects were enrolled in the study. Twelve subjects were enrolled in the study as normal volunteers in a protocol approved by Stanford's Panel on Human Subjects and were exposed to C fuscescens jellyfish. An additional 12 subjects were similarly in a protocol approved by the Institutional Review Board at Bert Fish Medical Center and were exposed to C quad-rumanus jellyfish. Participation in the study was limited to adults because it was not felt appropriate to expose children to stings, and participants were not expected to have any personal gain as a result of their participation in the study. Each subject signed an informed consent, met inclusion and exclusion criteria, and underwent a physical examination before participating in the study. Subjects were randomized in a double-blind fashion to receive application of either the inhibitor lotion (Safe Sea, Nidaria Technology Ltd, Jordan Valley, Israel) or conventional sunscreen to the left forearm followed by an application of the other lotion to the right forearm. Coppertone SPF 15 was used as the placebo sunscreen because it contains similar chemical components found in the inhibitor lotion save the active inhibitor. It is also waterproof and is a widely used sunscreen for recreational use.
An area of 18 X 6 cm was marked on each forearm, and the inhibitor lotion and placebo sunscreen were identically applied in a thin layer (routinely recommended application) to the forearms according to the randomization protocol. After the substances were allowed to dry for 10 minutes, two marks were made in the center of the application area at a distance of 3 and 5 cm for C quadrumanus and C fuscescens jellyfish, respectively.
Tentacles were removed from live jellyfish in storage tanks and held vertically in the air to allow excess water to drip off. The tentacles were placed on the skin with the lower end of the tentacles applied to the distal mark and 3 or 5 cm of tentacle placed in a straight line on the left forearm until it reached the proximal mark (Figure 4). The tentacle was left in contact with the forearm for 10 and 30 seconds for C quadrumanus and C fuscescens jellyfish, respectively, at which time it was removed with tweezers. The same protocol was repeated on the right forearm. If the subject experienced no discomfort in either arm during the first application, fresh tentacles were applied a second time to each arm for a total of 15 to 20 additional seconds. In the C fuscescens experiment, the tentacles were placed for an additional 15 seconds for a total of 60 seconds if the subject had again noted no discomfort.
Subjects were examined and queried about pain at 0, 15, 30, 60, 90, and 120 minutes after completion of the tentacle application. Pain was scored on a 0 (no pain) or 1 (pain) scale. If discomfort was noted in both arms, but one arm was less severe than the other, that arm was given a score of 0.5 to distinguish it from the arm with more discomfort. The degree of inflammation was evaluated by a dermatologist according to the following criteria : 0 (no change), 1 (skin color change only), 2 (edema), and 3 (blister or ulcer formation). These measurements were taken at the same time points.
Additionally, digital and 35 mm photographs were taken of each arm at 0 and 15 minutes (Figure 5). Statistical analyses were performed using SPSS for Windows 11.5 (SPSS, Chicago, IL). Mean pain and physician-assessed reaction were compared using 2-sided t tests. P values <.O5 are noted in the tables and text.
Results
In the group of subjects exposed to C fuscescens jellyfish, all 12 enrolled subjects completed the protocol. The mean application time of tentacles was 37.5 seconds, with a minimum time of 30 seconds and a maximum of 60 seconds (Table 1). All 12 arms pretreated with the placebo sunscreen demonstrated erythema, and all 12 subjects noted discomfort in that arm. In contrast, no arm pretreated with the jellyfish sting inhibitor had clinically evident skin changes (P < .01). Two subjects noted some discomfort in the arm treated with the sting inhibitor, and in both cases, this discomfort was rated as less than in the placebo-treated arm (P < .01).
Similarly, all 12 enrolled subjects completed the protocol in the group exposed to C quadrumanus jellyfish. The mean application time of tentacles was 15 seconds, with a minimum time of 10 seconds and a maximum of 30 seconds (Table 2). Of the 12 enrolled subjects, only 3 noted discomfort in the arm treated with the sting inhibitor lotion. The mean and median measures of discomfort were 0.3 and 0, respectively. In contrast, 10 subjects noted discomfort in the arm treated with placebo. The mean and median measures of discomfort were 0.83 and 1, respectively, for the placebo treated arms (P < .05). Additionally, on medical examination performed by a blinded physician and scored as previously described, there was evidence of a reaction in only one arm treated with the inhibitor lotion. The arms treated with the placebo demonstrated visible or palpable evidence of stings in 9 of 12 cases (P < .01).
Discussion
The jellyfish sting inhibitor lotion prevented clinical symptoms of the sting of the C fuscescens jellyfish in 10 of 12 subjects and diminished the pain of the jellyfish sting in the other 2 subjects. No visible sign of sting was noted in any of the arms treated with the inhibitor lotion, but erythema, edema, or both were present in all 12 arms treated with the placebo. The inhibitor also diminished the frequency and severity associated with the more severe sting of the C quadrumanus jellyfish. Three of 12 subjects experienced discomfort in the inhibitor-treated arm compared with 10 subjects in the placebo-treated arm, and only 1 inhibitor-treated arm had clinical evidence of a sting compared with 9 placebo-treated arms. As has been noted in previous experiments, the maximum discomfort associated with the sting of the jellyfish was not always apparent immediately after the sting but rather at a short time (approximately 15 minutes) later, and the clinical signs of the sting reached their maximum at approximately 30 minutes.
Stings from isolated jellyfish tentacles do not cause stings with the same frequency or intensity as live jellyfish in an open-water setting. It is possible that in an artificial setting, the effectiveness of the inhibitor might be overestimated because of an infrequency of stings. However, subjects did demonstrate signs and symptoms of the sting in all placebo-treated arms, suggesting that the jellyfish tentacles used in this study were capable of reliably causing stings and that some inhibition was clearly achieved. Anecdotal evidence suggests efficacy in open water, but future studies will be required to demonstrate efficacy in an underwater setting and after open-water contact with jellyfish. Additionally, whereas this inhibitor is formulated in a waterproof sunscreen, future research will be required to quantify the duration of protection afforded by the application of the inhibitor in an underwater setting.
In our study, the jellyfish sting inhibitor lotion did not completely inhibit all stings from the C fuscescens or C quadrumanus jellyfish, but it did significantly reduce the frequency and severity of these stings. C fuscescens (sea nettle) and C quadrumanus (sea wasp) species were used in this study because of their prevalence in the waters of the United States. However, the mechanism of action of the inhibitor lotion and the results of similarly designed trials suggest that the lotion is likely to be effective against several other species of jellyfish, including Rhopilema nomadica and Linuche unguiculata (one causative agent of seabather's eruption, also known as sea lice) (unpublished data, Nidaria Technology, 1999). Consequently, use of this jellyfish sting inhibitor lotion may be an important preventive measure for people who are exposed to stinging jellyfish in recreational and occupational settings.
Table 1. Results fiom C. fuscescens group comparing severity of reaction (including both pain and clinical signs of reaction) and time to reach reaction after contact with tentacles in inhibitor and placebo treated arms
| Inhibitor | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total contact | Max pain | Max | reaction | Max pain | Max | reaction | |||
| Subject N° | Time (s) | Amount of pain | Time (min) | Amount | Time (min) | Amount of pain | Time (min) | Amount | Time (min) |
| 1 | 45 | 0 | 0 | 0 | 0 | 1 | 15 | 2 | 15 |
| 2 | 30 | 0 | 0 | 0 | 0 | 1 | 60 | 1 | 30 |
| 3 | 30 | 0 | 0 | 0 | 0 | 1 | 30 | 2 | 30 |
| 4 | 30 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| 5 | 30 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| 6 | 30 | 0 | 0 | 0 | 0 | 1 | 30 | 2 | 30 |
| 7 | 45 | 0 | 0 | 0 | 0 | 1 | 30 | 2 | 30 |
| 8 | 45 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 120 |
| 9 | 60 | 0 | 0 | 0 | 0 | 1 | 30 | 1 | 30 |
| 10 | 45 | 0.5 | 15 | 0 | 0 | 1 | 0 | 2 | 30 |
| 11 | 45 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| 12 | 30 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| Median | 37.50 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 15.00 | 1.00 | 30.00 |
| Mean | 38.75 | 0.04 | 1.25 | 0.00 | 0.00 | 1.00 | 22.50 | 1.42 | 36.25 |
| P value | <.01 | <.01 | |||||||
Max. maximum ; Min, minimum.
Table 2. Results from C. qiiadvumawus group comparing severity of reaction (including both pain and clinical signs of reaction) and time to reach reaction after contact with tentacles in inhibitor and placebo treated arms.
| Inhibitor | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total contact | Max pain | Max | reaction | Max pain | Max | reaction | |||
| Subject N° | Time(s) | Amount of pain | Time (min) | Amoun | Time (min) | Amount of pain | Time(min) | Amount | Time (min) |
| 1 | 10 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 30 |
| 2 | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 90 |
| 3 | 20 | 0 | 0 | 0 | 0 | 1 | 15 | 2 | 120 |
| 4 | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 120 |
| 5 | 10 | 0 | 0 | 0 | 0 | 1 | 60 | 1 | 60 |
| 6 | 20 | 1 | 15 | 0 | 0 | 1 | 30 | 2 | 30 |
| 7 | 10 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 8 | 10 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 20 | 0 | 0 | 0 | 0 | 1 | 30 | 1 | 30 |
| 10 | 20 | 1 | 15 | 1 | 90 | 0 | 0 | 0 | 0 |
| 11 | 30 | 0 | 0 | 0 | 0 | 1 | 60 | 2 | 60 |
| 12 | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| Median | 10 | 0 | 0 | 0 | 0 | 1 | 15 | 1 | 30 |
| Mean | 15 | 0.3 | 2.5 | 0.1 | 7.5 | 0.83 | 20 | 1 | 47.5 |
| P value | <.05 | <.01 | |||||||
Max. maximum ; Min. minimum.
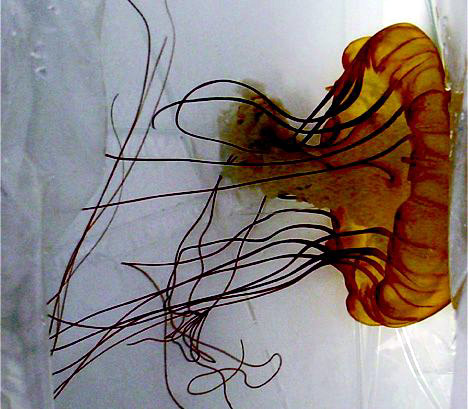
Figure 1. Chrysaora fuscescens jellyfish.
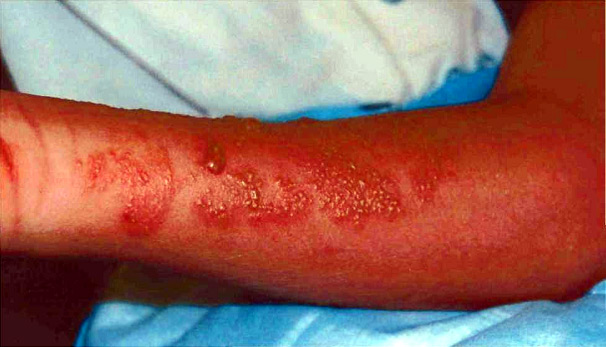
Figure 2. Thirteen-year-old surfer a day after a sting from a Chiropsalmus quadrumanus jellyfish.
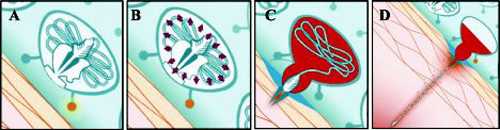
Figure 3. Jellyfish stinging mechanism. A, Stimulation from the skin initiates the stinging cell discharge process. B, High internal pressure of 150 atm develops within the stinging capsule. C, With acceleration of 40 000 times the force of gravity, the shaft drills a hole into the skin. D, A tubule follows the shaft and injects poison into the body.
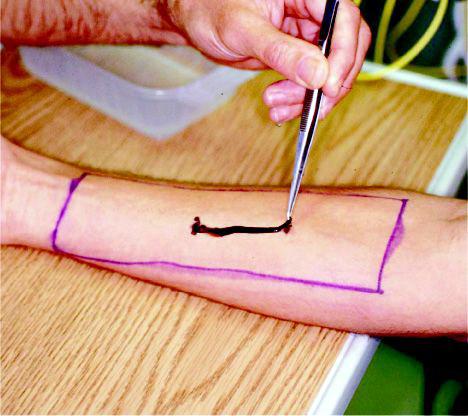
Figure 4. Application of Chrysaora fuscescens tentacles to the arm of a subject within the region treated with either placebo or a jellyfish sting inhibitor lotion.

Figure 5. Erythema is notable on the arm treated with the placebo lotion and absent on the arm treated with the inhibitor lotion after exposure to a jellyfish tentacle.
References
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1984 ;21:527-555.
- McGoldrick J, Marx JA. Marine envenomations, II : invertebrates. J Emerg Med. 1992 ; 10:71-77.
- Fisher AA. Aquatic dermatitis, I : dermatitis caused by coelenterates. Cutis. 1999 ;64:84-86.
- Brown CK, Shepherd SM. Marine trauma, envenomations, and intoxications. Emerg Med Clin North Am. 1992 ;10 : 385-407.
- Burke WA. Cnidarians and human skin. Dermatol Ther. 2002 ;15:18-25.
- Auerbach PS. Marine envenomations. N Engl J Med. 1991 ;325:468-493.
- Haddad V, da Silveira FL, Cardoso JLC, Morandini AC. A report of 49 cases of cnidarian envenoming from southeastern Brazilian coastal waters. Toxicon. 2002 ;40:1445-1450.
- Bengston K, Nichols MM, Schnadig V, Ellis MS. Sudden death in a child following jellyfish envenomation by Chiropsalmus quadrumanus : case report and autopsy findings. JAMA. 1991 ;266:1404-1406.
- Tucker J. Coelenterate and jellyfish envenomations. In : James WD, Elston D, eds. eMedicine Dermatology. St. Petersburg : eMedicine Corporation ; 2002. Available at : http://www.emedicine.com/EMERG/topic104.htm. Accessed April 5, 2004.
- Lotan A, Fishman L, Loya Y, Zlotkin E. Delivery of a nematocyst toxin. Nature. 1995 ;375:456.
- Holstein T, Tardent P. An ultrahigh-speed analysis of exocytosis : nematocyst discharge. Science. 1984 ;223:830-833.
- Lotan A, Fishman L, Hillel RB, Loya Y, Zlotkin E. Toxinology and ecology of the Mediterranean jellyfish Rhopilema nomadica. Biochemical aspects and marine pharmacology. In : Lazarovici P, Spira M, Zlotkin E, eds. Biochemical Aspects of Marine Pharmacology. Fort Collins CO : Alken Inc ; 1996:132-145.
- Burnett JW, Calton GJ. Jellyfish envenomation syndromes updated. Ann Emerg Med. 1987 ;16:1000-1004.
- Lotan A, Fishman L, Zlotkin E. Toxin compartmentation and delivery in cnidaria : the nematocyst's tubule as a multiheaded poisonous arrow. JExp Zool. 1996 ;275:444-451.
- Burnett JW, Calton GJ. Venemous pelagic coelenterates : chemistry, toxicology, immunology and treatment of theirstings. Toxicon. 1987 ;25:581-602.

























